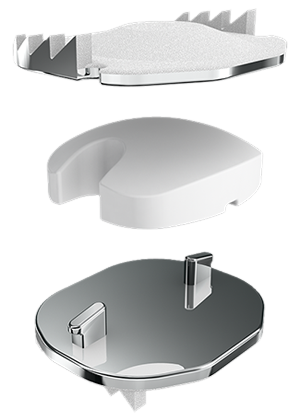
The Mobi-C disc has three parts: two metal plates and a plastic insert in the middle. The plates are made of a mix of metals commonly used in spine surgery (cobalt, chromium, and molybdenum).
The plates have teeth on the top and bottom that help hold the plates to the vertebrae. The teeth are pressed into the bone with no bone cut out, which makes the Mobi-C design and technique bone sparing.
The outside of the metal plates are sprayed with a coating (hydroxyapatite). This coating helps the vertebrae to grow and attach to the metal plates for long term stability.
The plastic insert is made from polyethylene. The insert is flat on the bottom and round on the top. The insert is made to move as you move your neck.