Mobi-C® Cervical Disc: FDA Approved For Use at Both One and Two Levels
In 2013, Mobi-C was approved in the United States by the Food and Drug Administration (FDA) for use at one and two levels. Zimmer Biomet estimates that 1 out of 3 cervical disc patients are indicated for two-level replacement. Mobi-C is the first cervical disc with FDA approval for use at two levels in the U.S.
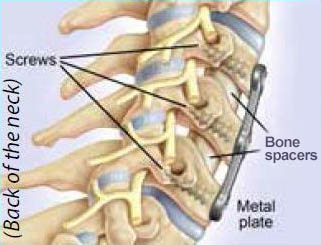
Fusion
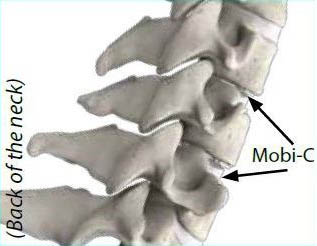
Mobi-C
Anterior Cervical Discectomy and Fusion
Before artificial discs, most often a patient would get an anterior cervical discectomy and fusion (ACDF). In this fusion surgery, the doctor removes the unhealthy disc. The empty disc space is filled with a bone spacer or plastic implant. The implant helps match the disc height to the levels above and below. Restoring the disc height can help remove pressure on the nerves and/or spinal cord. Then, typically a metal plate with screws is placed on the front of the neck.
Generally, ACDF surgery achieves successful outcomes with acceptable complication rates.1-2 However, the ACDF process stops the natural motion at the surgery level(s). This is widely accepted to cause:
- Spine movement at the levels adjacent to the surgery level beyond the normal range of motion (hypermobility)3-6
- Increased degeneration of adjacent levels after surgery7-9
Cervical Disc Replacement – the Non Fusion Option
Like ACDF, cervical disc replacement removes the unhealthy disc and helps match the disc height to the levels above and below. Like ACDF, cervical disc replacement restores the disc height and can help remove pressure on the nerves and/or spinal cord. However, cervical disc replacement is also designed to maintain normal spinal motion at the surgery level(s).
Results from the U.S. Mobi-C FDA Two-Level Study
In the U.S. clinical study, patients treated with Mobi-C were compared against patients treated with ACDF at both one and two levels. Mobi-C implanted at one level demonstrated equivalent outcomes compared to ACDF at one level, and Mobi-C two-level patients achieved superior outcomes to ACDF five years after surgery.
Am I a candidate for cervical disc replacement with the Mobi-C?
Five years after surgery, key results in the two-level study showed:
- Of Mobi-C patients, about three quarters (84% or 155 out of 184 patients) were able to bend their head forward to backward (flexion-extension) either the same amount or more at five years as before they were treated based on the combined motion at both operated levels.
- The rate of major complications was similar for Mobi-C patients (17% or 38 out of 225 patients) and ACDF patients (22% or 23 out of 105 patients).
- Major complications included: diminished neurologic status, spontaneous fusion in the Mobi-C group or failure to fuse in the ACDF group, and adverse events determined to be major complications and related to the study device.
- Another surgery at the treated level was necessary in more ACDF patients (16% or 17 out of 105 patients) than Mobi-C patients (4% or 9 out of 225 patients).
- For two-level cases, Mobi-C patients returned to work on average in 45.9 days, compared to ACDF patient at 66.8 days. The average return to work time was 20.9 days shorter for Mobi-C compared to ACDF.
The patients in this study will continue to be followed for 7 years after surgery. The clinical benefit beyond five years has not been measured.
To find a trained Mobi-C surgeon, please visit the surgeon locator.
Potential adverse effects from surgery with the Mobi-C
Throughout the FDA clinical study, patients reported health related problems to their doctors. Some of the more common study events for two-level Mobi-C and the ACDF patients include:
| Adverse Effect |
Mobi-C |
ACDF |
| Neck pain |
39% (90 out of 234 patients) |
57% (60 out of 105 patients) |
| Arm (extremity) pain |
23% (54 out of 234 patients) |
30% (31 out of 105 patients) |
| Back pain |
32% (76 out of 234 patients) |
30% (31 out of 105 patients) |
| Shoulder pain |
27% (64 out of 234 patients) |
41% (43 out of 105 patients) |
| Feeling of pins and needles in the arms (sensory disturbance) |
41% (96 out of 234 patients) |
52% (55 out of 105 patients) |
| Difficulty swallowing (dysphagia) |
15% (36 out of 234 patients) |
23% (24 out of 105 patients) |
| Headache |
25% (58 out of 234 patients) |
25% (26 out of 105 patients) |
Nine (4%) treated with Mobi-C and 17 patients (16%) treated with ACDF had additional surgery at the same level within 5 years after their surgery. Seven patients (3%) treated with Mobi-C and twelve patients (11%) treated with ACDF, had surgery at an adjacent level within 5 years after surgery. No mechanical failures of the Mobi-C device were observed in any study patients.
Please ask your doctor for more information about any additional risks that could be related to the surgery. For a complete list of risks and benefits associated with cervical spine surgery with Mobi-C, please visit www.cervicaldisc.com/aboutyourneck.